LiFePO4 stands for Lithium iron phosphate and may sometimes refer to as Li-IP or LFP. It is a specific kind of rechargeable lithium-ion battery made out of Lithium iron phosphate. Today, LiFePO4 is regarded as the safest, most reliable lithium battery.

Compared to lithium-ion polymer batteries, the cycle life of the LiFePO4 battery is over 4 times longer.
These batteries use graphitic carbon electrodes as the anode and LiFePO4 as the cathode.
The two electrodes are separated by a polymer membrane. The membrane is made from a particular form of polymer (plastic) that includes many small microscopic pores to make it simple for the lithium ions to move through. This would not allow the flow of electrons but the Lithium ions.
History of the Development of LiFePO4 Battery
LiFePO4 was first discovered in 1950 by Destenary. It was in the minerals of triphylite and lithuophilite.
The petroleum crisis in the early 1970s triggered world wild research interest to develop energy storage technologies. Among the different materials, Li-ion (LIB) based materials are the most researched area for battery development.
In 1996, Goodenough and co-workers were able to understand the electrochemical extraction and insertion process of Li from LiFePO4.
Starting on this point, direct research on LiFePO4 has been done extensively. The scientists were able to find numerous advantages of LiFePO4 which helps to build a safe and efficient battery for the industry.
Regarding the commercialization of LiFePO4 into battery products, BYD company devoted extensive efforts during the period around 2003. Eventually, they were able to produce LiFePO4 batteries for the market as the leading manufacturer.
What is the Cathode Material/s for LiFePO4 Battery?
When it comes to the general category of Li-ion batteries, numerous materials can be used as electrodes. Accordingly, various types of lithium batteries are:
- Lithium Iron Phosphate (LiFePO4)
- Lithium Cobalt Oxide (LiCoO2)
- Lithium Titanate (LTO)
- Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2)
- Lithium Manganese Oxide (LiMn2O4)
However, LiFePO4 batteries use, LiFePO4 as the cathode. Using an iron-based material for the cathode is more appealing because it is cheap, abundant, and less toxic.
Additionally, it has advantages from thermal stability, extremely flat potentials (3.4 V) during charge and discharge processes, a high theoretical capacity (170 mAhg-1 or 610 C/g), and long cycle life.
LiFePO4 as the cathode also experiences some issues: low tapped density and poor low-temperature performance.
However, there is new research undergoing to overcome these drawbacks as well. Olivine LiFePO4 cathode material is one such research approach that shows promising results.
How do Lithium Iron Phosphate Batteries Work? (Charging and Discharging)
Charging Process of LiFePO4 Battery
In the anode phosphate and iron, ions form a grid-like structure whereas the lithium ions are weakly tapped in between them. When the battery gets charged, those Lithium ions are pulled through the polymer membrane to reach the negative electrode, where they are captured and held.
When every positive lithium ion present in the cathode terminal moves to the anode terminal and is held appropriately between layers of graphene, the battery will be fully charged.
The charging process can be expressed in this equation well.
LiFePO4-x Li+-xe- → xFePO4+1-x LiFePO4
Discharging Process of LiFePO4 Battery
As was previously mentioned, during a LiFePO4 battery’s charging cycle, the positive lithium ions that have been released from the positive electrode flow through the electrolyte to the negative electrode and are stored there.
When the battery is connected to an electrical load, those positive Lithium ions move back to the positive electrode through the polymer membrane.
At the same time, the electrons flow through the load powering the load and discharging the battery. When all the Lithium ions are pulled back to the cathode the battery is considered fully discharged.
The discharging equation of the LiFePO4 battery is,
FePO4+ x Li++xe- → x LiFePO4+(1-x) FePO4
Further, the LiFePO4 battery discharge process can clearly understand through a battery discharge curve as shown below.
The below discharge graph shows how single-cell LiFePO4 batteries lose their energy over time under different temperature levels.
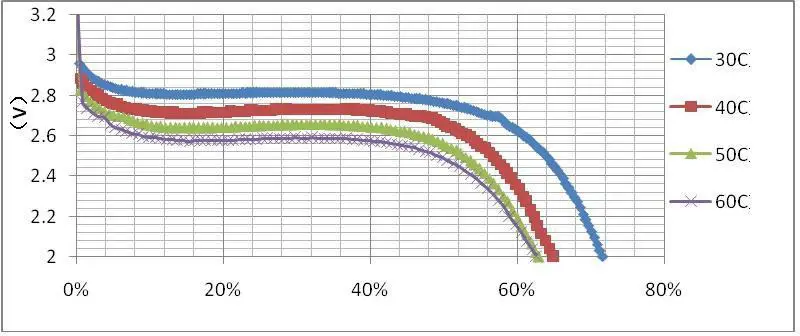
If you like to learn in-depth about the charge and discharge behavior of LiFePO4 batteries, then I’m suggesting you look into the LiFePO4 cyclic voltammetry studies such as “Study of LiFePO4 by Cyclic Voltammetry” done by Denis Y W Yu and team.
Applications of LiFePO4 Batteries
Uses In Energy Storing (especially in Solar Systems)
LiFePO4 batteries are widely used in large-scale electric energy storage. It has a promising future in the sectors of secure grid connections for renewable energy power plants, peak power grid regulation, UPS power supplies, and emergency power supply systems.
It is possible to pair LiFePO4 batteries with a solar panel and solar controller. The LiFePO4 battery can be charged whilst the electrical load is powered by the solar panel.
Uses In Automobiles
Modern low-speed vehicles such as passenger cars, buses, and some logistic vehicles and low-speed electric vehicles use LiFePO4 batteries. Lithium iron phosphate batteries continue to be popular in the field of passenger automobiles, while their share in the field of special-purpose vehicles is rapidly rising.
By 2030, the market for LiFePO4 batteries is expected to reach $9.9 billion.
The principal factors driving the desire for lithium-iron phosphate batteries are the rising demand from the automotive industry, especially electric vehicles and innovative breakthroughs in lightweight materials.
Also, the new LiFePO4 prismatic batteries are somewhat different from the traditional shape of the battery. These LiFePO4 prismatic cells are optimized specially for the automobile industry and they’re getting quite popular.

Further, LiFePO4 batteries are been used for the electric boats as the main power source too.
When these are used with fishing boats and because of the reduced charging time and longer duration, you can spend more time on the water. A speed boost and improved handling are provided by less weight of the boat.
Which cars have LiFePO4 batteries?
Tesla Model S, Volkswagen e-Golf, Ford Fusion, Nissan Leaf, Porsche Panamera, and BMW 740iL have LiFePO4 batteries.
Uses In the Startup power supply
The lithium iron phosphate battery is capable of producing high power instantly making them more suitable for a starter battery. Along with the slow start and stop functions, it also has the ability of taxiing, recover braking energy and increase acceleration power.
Advantages and Disadvantages of LiFePO4 Batteries.
Now, let’s look into both advantages and disadvantages of LiFePO4 batteries.
Advantages of LiFePO4 Batteries
- Safety Operation
The lithium iron phosphate crystal consists of strong P-O bonds, thus stable and hard to decompose. Additionally, LiFePO4 batteries do not heat or produce any oxidizing substances even at high temperatures.
- Long Battery Cycle Life
The cycle life of the lithium iron phosphate power battery is more than 2000 cycles and can reach 5000 cycles. You may use the regular charge a maximum of 2000 times.
So simply it means LiFePO4 has a good life expectancy.
- High Charging and Discharging Efficiency
In comparison to lead-acid batteries, which have a charge and discharge efficiency of roughly 80%, lifepo4 batteries have a high charge and discharge efficiency that can reach over 90% when discharged.
- Environmentally Friendly
Since the electrodes of LiFePO4 batteries are made of non-hazardous materials, they pose significantly smaller harm to the environment than lead-acid batteries do.
- Temperature Performance
The maximum temperature of lithium iron phosphate is between 350 and 500°C and it has a wide operational temperature range (-20–+75 °C). This will enable a wide range of applications.
- No Memory effects
The batteries quickly lose useful capacity if they are frequently recharged after being just partially depleted because of a lower working voltage. Unlike other types of batteries, lithium-ion batteries are considered to have no memory effect.
Disadvantages of LiFePO4 Batteries
- Low energy density
An LFP battery has a little lower energy density than a lithium-ion battery. Their energy content ranges from 90 to 165 Wh/kg.
Because of this, these batteries are unable to find in more compact, power-intensive applications.
- Low tap density
Because of their low tap density, LiFePO4 batteries cannot be used in compact, portable electronics like smartphones. Therefore, the majority of LiFePO4 batteries are used as power batteries for EVs, LEVs, and electric motorcycles.
- Poor low-temperature performance
LiFePO4 batteries perform poorly when being discharged at -20°C. But lithium-ion or lithium-polymer batteries can discharge even at -40 °C.
Lithium-ion or LiFePO4 Batteries
These two types of lithium batteries have significant differences in every aspect. To understand the specific difference between LiFePO4 and Li-ion batteries, you can refer to the below table.
| Lithium-ion | LiFePO4 | |||
| Energy density | high | 100-265 Wh/kg | low | 90-165 Wh/kg |
| Cycle life | low | 300 – 500 cycles | high | about 3000 cycles |
| Depth of Discharge (DoD) | low | 80% – 95% | high | 100% |
| Weight | high | low | ||
| Thermal Stability | low | High | 100% incombustible |
Lithium-ion batteries often weigh around 50% less than lithium-ion batteries of the same capacity.
LiFePO4 is a preferable choice in every relevant area. You get higher performance, better value, and a significantly longer lifespan with these batteries.
When you take into account the energy density, the lithium-ion equivalent is the only one that stands out more.

Eng. Matthew Joseph Nandirio is the Founder of walkingsolar.
After graduating from the University of Houston in 2002, matt started working as a Solar Electrical Engineer for several multi-national solar energy companies.
He has a wide range of experiences including solar system requirement analysis, planning, maintaining, debugging and even solar device development through research.
He now shares his 20 years of expertise through his articles on the walkingsolar website.
Further, he is also the author of two books on Solar Technology, “Solar Power for Villages” and “DIY Solar System for Dummies”.
